The Little Country That Could
Cuba’s COVID-19 saga wasn’t much touted, because, what the hell, who cares about a Communist relic, and what could they be able to accomplish? In truth, the current epidemiological situation in Cuba isn’t good, with new daily infections close to 7,000. And yet, they did a lot in Cuba, including the vaccination with at least one dose of 6.8M (official population 11.33M). Vaccination with what, exactly?
Good question. Everyone was vaccinated with one of the two vaccine candidates, as only on July 9 one of the candidates, Abdala, received an emergency use authorization.
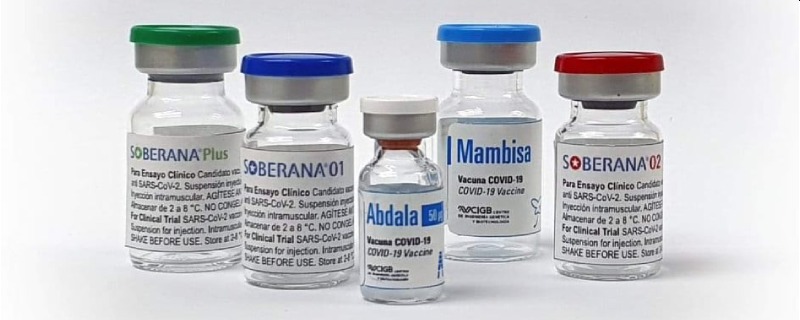
The history of the efforts of “the small island that could” includes five vaccine candidates: Mambisa, Abdala, Soberana 01, Soberana 02, and Soberana Plus. Of them, Mambisa and Soberana 01 left the stage rather quickly, and the three others evolved towards two vaccination schemes:
- Soberana 02, in two doses: with an efficacy of 62%, it couldn’t carry on.
- Soberana 02 (2 doses at 14 days) + Soberana Plus (as a third dose, after 14 more days): efficacy 91.2%, in evaluation for authorization.
- Abdala, in three doses: efficacy 92.28%, just authorized in Cuba, after having been sent to Venezuela even before any authorization!


The composition of the aforementioned vaccines is as follows:
- Soberana 02: Monomeric SARS-CoV-2 RBD (receptor-binding domain of the spike protein) protein, chemically conjugated to tetanus toxoid, with added aluminum hydroxide, for intramuscular administration.
- Soberana Plus: Dimeric SARS-CoV-2 RBD protein, with added aluminum hydroxide, for intramuscular administration.
- Abdala: Based on the monomeric SARS-CoV-2 RBD protein, with added aluminum hydroxide, for intramuscular administration.
Of course, the Western medical authorities are circumspect if not skeptical, as these results haven’t been validated outside Cuba. However, here you have two relevant studies:
- Relevant to Soberana 02: SARS-CoV-2 RBD-Tetanus toxoid conjugate vaccine induces a strong neutralizing immunity in preclinical studies (doi.org/10.1101/2021.02.08.430146)
- Relevant to Abdala: The SARS-CoV-2 receptor-binding domain expressed in Pichia pastoris as a candidate vaccine antigen (doi.org/10.1101/2021.06.29.21259605)
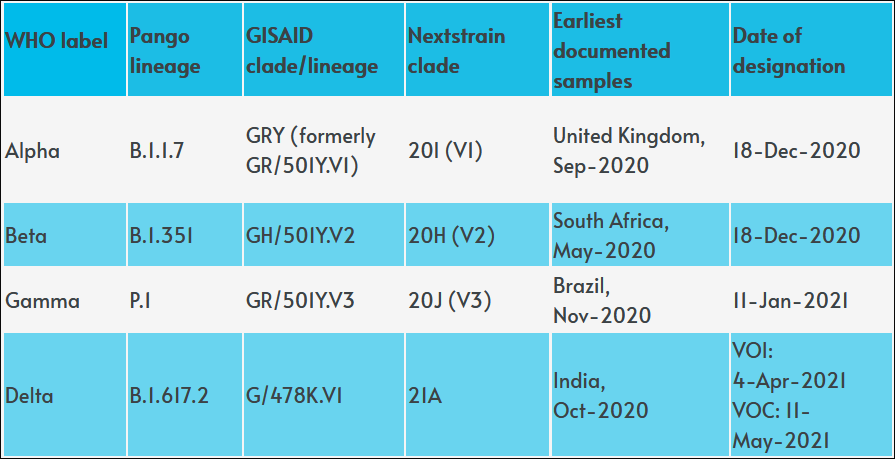
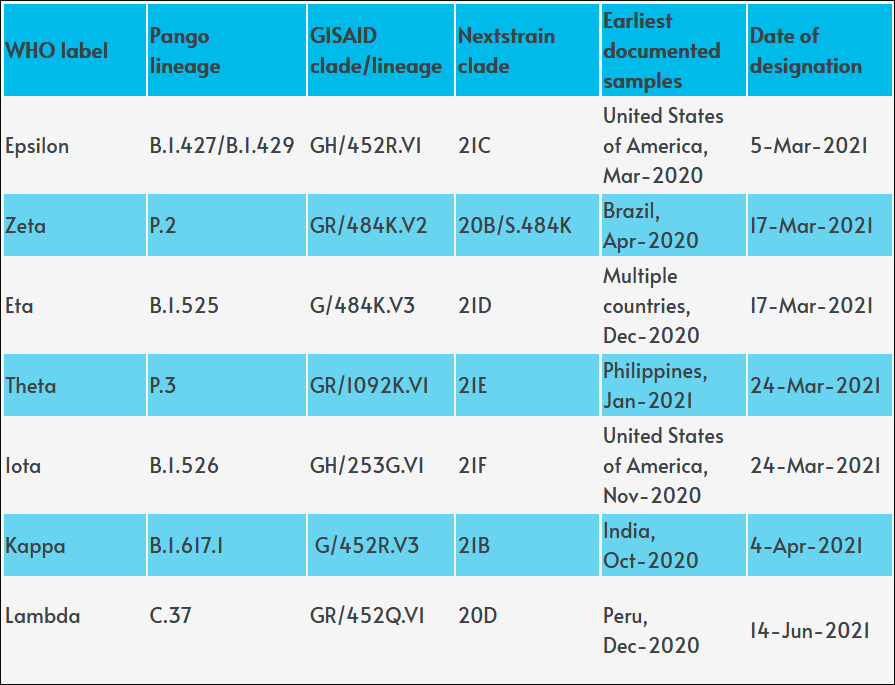
Meanwhile, Lambda seems to have evolved from a “variant of interest” to a “variant of concern”: here’s what you need to know. Let me remind you the COVID-19 alphabet:

Leave a Reply